What Is the Conjugate Acid of No3
From Bronsted Lowry theory we can conclude that the conjugate base of HPO4 - 2 is PO4-3. What is the conjugate acid for NH2-Recommended textbook explanations.

Define Add And Base By Bronsted Lowry Concept Identify A Conjugate Acid Base Pair In The Following Hno3 Aq H2 1 Rarrh3o Aq No3 Aq
It is the weak conjugate base of Strong Acid HNO3.

. Part 3 - If the concentration of H3O is 35 10-3 M the concentration of OH is _____ M. Therefore NH4 is the conjugate acid of ammonia. What is the conjugate acid of NO 3-.
NO3- ions will not affect the pH. The Nitrate ion has the formula NO3-. Hence an acid donates a proton to form its corresponding conjugate base while a base accepts a proton to form its corresponding conjugate acid.
Show your Calculation Part 4- Identify each substance as an. The conjugate acid if NO3 -1 is HNO3. NO3 SH TsO-HCO3 N O O-10-9-8-36-24-17-13 47 48 32 sulfuric acid hydroiodic acid hydrobromic acid protonated ether protonated alcohol hydronium ion nitric acid hydrofluoric acid hydrogen nitride carboxylic acids protonated ketone-73 637 7 carbonic acid tosic acid -06 protonated pyridine 52 pKa Chart conjugate acid conjugate base.
A conjugate acid is a substance that is formed when a base receives a proton or H. TABLE OF CONJUGATE ACID-BASE PAIRS Acid Base K a 25 oC HClO 4 ClO 4 H 2 SO 4 HSO 4 HCl Cl HNO 3 NO 3 H 3 O H 2 O H 2 CrO 4 HCrO 4 18 x 101 H 2 C 2 O 4 oxalic acid HC 2 O 4 590 x 102 H 2 SO 3 SO 2 aq H 2 O HSO 3 171 x 102 HSO 4 SO 4 2 120 x 102 H 3 PO 4 H 2 PO 4 752 x 103 FeH 2 O 6 3 FeH 2 O 5 OH 2 184 x 103 H 2 C 8. We review their content and use your feedback to keep the quality high.
Similarly NH4 loses a proton to give conjugate base NH3. What is the conjugate acid of co32. Both mass and charge are conserved.
NH4 aka ammonium ion is for all practical purposes an acid. So add a H unit to NH_3 and I gets NH_4 ammonium ion. Is NO3- a weak base.
In this example that conjugate base is the nitrate. By the same procedure if I remove H from any species I get the conjugate. In most cases the acid molecule that remains after losing a hydrogen ion is an acids conjugate base.
What is the conjugate base for H2SO3. Adding a hydrogen ion H1 to the nitrate ion gives us the conjugate acid nitric acid. Part 1- What is the conjugate acid of H2PO4.
Conjugate acid of N-3 is NH-2 that of NH2-1 is NH3 that of NH3 is NH41 and HCOO-1 is HCOOH. Adding a H to the given species gives its conjugate aci. Basically NH4 H2O - NH3 H3O.
Are both mass and charge conserved here. NH4 is the conjugate acid of the base NH3 aka ammonia. The conjugate acid if NO3 -1 is HNO3.
Who are the experts. Part 2 -Write the balanced chemical equation for the neutralization reaction between SrOH2 and H3PO4. This means the resulting conjugate acid of CO32 is A HCO3.
NH4 is the acid because it donates an H ion to the water. The conjugate acid if NO3 -1 is HNO3. Here NH3 is the conjugate acid of Bronsted base NH2-1.
The conjugate acid of nitric acid would be H2NO3 which is a very unstable species but is produced in the nitration of aromatic compounds. Adding a hydrogen ion H1 to the nitrate ion gives us the conjugate acid nitric acid. What is the salt.
HCO3 H H2CO3. What is the conjugate base of NH4. Experts are tested by Chegg as specialists in their subject area.
The pH of a solution is 629 - 005. Is NO3 conjugate acid. The formula of the conjugate acid is the formula of the base plus one hydrogen ion.
What is the conjugate acid for NO3-what is the conjugate base for H3O what is the conjugate acid for H2O. It readily gives off a proton in the form of h. The conjugate acid of ammonia is the ammonium ion NH_4.
Nitrate or NO3- is the conjugate base of HNO3. Carbonate ions are obtained from weak acids H2CO3 and HCO3- so the conjugate base CO32- wil l be strong and accepts protons from water. Ammonia or NH3 is a base.
4 rows What is the conjugate acid of no3. Because when a Bronsted base takes up a proton it is converted to its conjugate acid. A NO3- B HPO42- C Br- D S2- 43356 results.
Pearson Chemistry Matta Staley Waterman Wilbraham. Nitrate or NO3- is the conjugate base of HNO3. The conjugate acid of any species is the original species PLUS a proton H.
As a result CO32 becomes HCO3. It is the conjugate base of Nitric acidHNO3. What is the conjugate acid of no 3.
Adding a hydrogen ion. How do you find the conjugate acid. Write the conjugate acid for the following.
It accepts a proton to give its conjugate and NH4. The reaction resulting in the conjugate base of HNO3 is HNO3 H2O H3O NO3-. Carbonic acid or H2CO3 will be the conjugate acid of hydrogen carbonate.
Being the conjugate base of nitric acid a strong acid thenitrate ion is a weak base.

Acids Bases And Salts Learning Activities Distance Learning Chemistry Activities Common Core Reading Common Core State Standards

Acids Bases And Salts Learning Activities Distance Learning Chemistry Activities Common Core Reading Common Core State Standards

Solved 1 The Formula For The Conjugate Acid Of No3 Is 2 Chegg Com
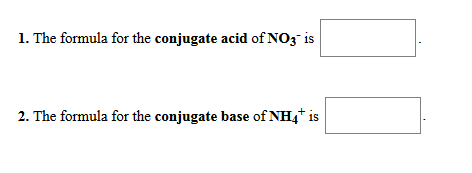
Solved 1 The Formula For The Conjugate Acid Of No3 Is 2 Chegg Com

Relative Strengths Of Some Common Conjugate Acid Base Pairs Listed Download Scientific Diagram

Conjugate Acid Base Pairs Video Khan Academy
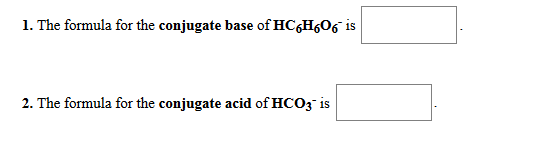
Solved 1 The Formula For The Conjugate Acid Of No3 Is 2 Chegg Com

Oxidation Reduction Reactions Redox Teaching Chemistry Chemistry Classroom Electrochemistry

Pin On Test Bank Cognition 4th Edition
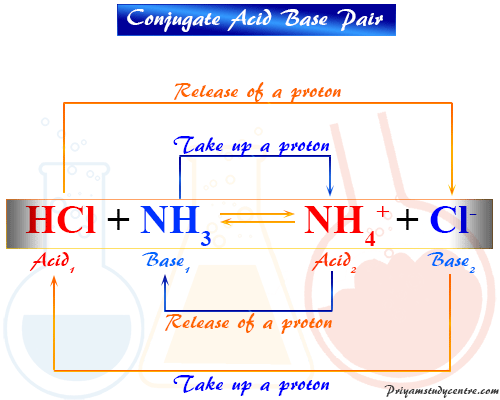
Conjugate Acid Base Pair Definition Concept Examples List

5 1 Acid Base Definitions Conjugate Acid Base Pairs General Chemistry For Gee Gees

Welcome To Learnapchemistry Com Ap Chemistry Teaching Chemistry Ap Chem

Solubility Rules Mnemonic Teaching Chemistry Chemistry Lessons Chemistry Education






Comments
Post a Comment